
Platinum resistance is a multifactorial process that has been extensively investigated 7. Apoptosis eventually occurs when damage exceeds repair. This ultimately generates DNA lesions, followed by activation of the DNA damage response (DDR) 5, 6. Platinum compounds covalently bind DNA, generating protein–DNA or DNA–DNA complexes, along with inter- and intra-strand adducts 5, 6. Circumventing this pervasive problem is an unmet clinical challenge. Despite this aggressive approach, 85% of patients will subsequently relapse and sequential platinum treatment inevitably results in chemotherapy resistance 4. Standard treatment is still reliant on combination surgery and platinum-based chemotherapy 3. Ovarian cancer is the seventh most common cancer type amongst women 1 and the most common histological subtype is high-grade serous cancer (HGSC) 2. Since chloroxine is already licensed, this exciting combination therapy could now be rapidly translated for patient benefit. In conclusion, our novel results show that chloroxine facilitates platinum-induced DNA damage to restore platinum sensitivity in HGSC. In contrast, treatment with chloroxine and carboplatin combined, was able to maintain tumour volume at baseline for over 4 months.
Finally, in our platinum-resistant, intraperitoneal in vivo model, treatment with carboplatin alone resulted in a transient tumour response followed by tumour regrowth. Moreover, this unrepaired DNA damage was associated with p53 stabilisation, cell cycle re-entry and triggering of caspase 3/7-mediated cell death. Co-treatment with carboplatin and chloroxine (but not either drug alone) caused an increase in γH2AX expression, followed by a reduction in platinum-induced RAD51 foci. Further mechanistic investigation revealed that chloroxine overrides DNA damage tolerance in platinum-resistant HGSC. Synergy was independent of chloroxine’s predicted ionophore activity and did not relate to platinum uptake as measured by atomic absorption spectroscopy. In extensive validation experiments, chloroxine restored sensitivity to both cisplatin and carboplatin, demonstrating broad synergy in our range of experimental models of platinum-resistant HGSC. We performed a drug screen with 1177 FDA-approved drugs and identified the hydroxyquinoline drug, chloroxine. Although initially sensitive to platinum chemotherapy, resistance is inevitable and there is an unmet clinical need for novel therapies that can circumvent this event. Our results highlight PoVS can be a promising treatment strategy for eliminating CML cells and LSCs selectively, with the reduced ponatinib doses.High-grade serous cancer (HGSC) accounts for ~67% of all ovarian cancer deaths. Transcriptional regulation resulted in alterations in the expression levels of target mRNAs. The subsequent downstream effects were an increase in C/EBP transcriptional activity and decreases in activities of E2F/DP1, Myc/Max, CREB, STAT3, NFκB, AP-1, Elk-1/SRF. PoVS treatment inhibited PI3K/Akt/mTOR pathway more consistently than either of the two agents alone through reducing p-Akt, p-mTOR, p-S6K, p-PRAS40, p-S6. VS-5584 reduced the ponatinib dose required to target leukemic cells. Moreover, ponatinib and VS-5584 mediated synergistic anti-leukemic effects on leukemic cells. Normal hematopoietic stem cell line was the least affected. VS-5584 inhibited the PI3K/Akt/mTOR pathway, resulting in reduced cell viability, slightly induced caspase-independent apoptosis, prominent G 0/G 1 cell-cycle blockade that is not a consequence of quiescence.

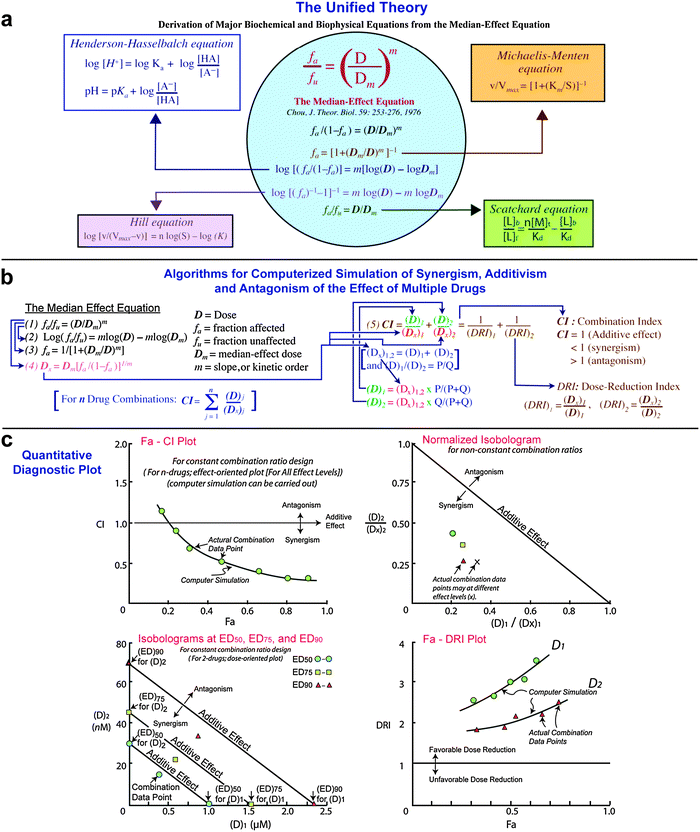
VS-5584 exerted selective cytotoxic effects against CML and LSC cell lines. We investigated the effects of inhibitory concentrations on apoptosis, cell viability and cell-cycle regulation (flow cytometry), protein levels (ELISA, Western blot), transcriptional activities (dual-luciferase reporter assay), gene expressions (qRT-PCR). We measured the cytotoxicities of ponatinib, VS-5584, and PoVS (CCK-8 assay), and used the median-effect equation for combination analyses. We evaluated the potential of ponatinib and PI3K/mTOR dual-inhibitor VS-5584 combination (PoVS) therapy to increase the anti-leukemic effects of ponatinib and investigated the underlying mechanisms at the molecular level. Ponatinib is used for advanced treatment of chronic myeloid leukemia (CML), although low doses to prevent side effects do not suppress survival pathways and eradicate leukemia stem cells (LSCs).


 0 kommentar(er)
0 kommentar(er)
